-
The disease in numbers
The damaged process happens through the excessive deposition of collagen and other connective tissue proteins, which cause progressive scarring of lung tissue through inflammation and fibrosis. This disorganized tissue remodeling impairs the vital role of the lungs in respiration with devastating consequences in terms of functional capacity, quality of life and increased mortality.1The prevalence of ILD has been estimated as 81/100,000 in males and 67/100,000 in females.2
Within ILD, the most common classification is Idiopathic Pulmonary Fibrosis (IPF). In Europe, IPF prevalence ranged from 1.25 to 23.4 cases per 100,000 population.3
Furthermore, ILD occurs in 15% of patients with connective tissue disease (CTD), which is a group of autoimmune diseases from which the most common, rheumatoid arthritis (RA), affects between 0.5% to 2% of the general population in the USA.4
![]()
-
Diagnostic pathways
The current diagnostic pathway involves several steps:- Physical examination (i.e. cardiopulmonary exercise test, six-minute walking test);
- Pulmonary function tests like Forced Vital Capacity (FVC) and gas exchange (i.e. diffusing capacity of the lung for carbon monoxide (DLCO));
- Imaging Tests like X-rays and HRCT (Computerized tomography scans);
- In case of still insufficient diagnosis Bronchoalveolar lavage (BALf) and/or Biopsy are performed
The most relevant ILDs may be associated with a progressive fibrosing phenotype, which leads to link this group of ILDs with a term known as progressive-fibrosing ILD. Among these progressive-fibrosing ILDs are: idiopathic pulmonary fibrosis (IPF), hypersensitivity pneumonitis (HP), Autoimmune ILDs (CTD-ILD), Sarcoidosis and idiopathic interstitial pneumonias (IIPs). All these ILDs share a progressive-fibrosing feature irrespective of the trigger for the fibrosis.5
Owing to this new approach, it has become critical to rapidly identify patients whose disease will progress to extensive lung fibrosis, and for this reason, the use of serum biomarkers can be of added value in disease activity assessment and prediction of the disease.6
-
Krebs von den Lungen 6 (KL-6)
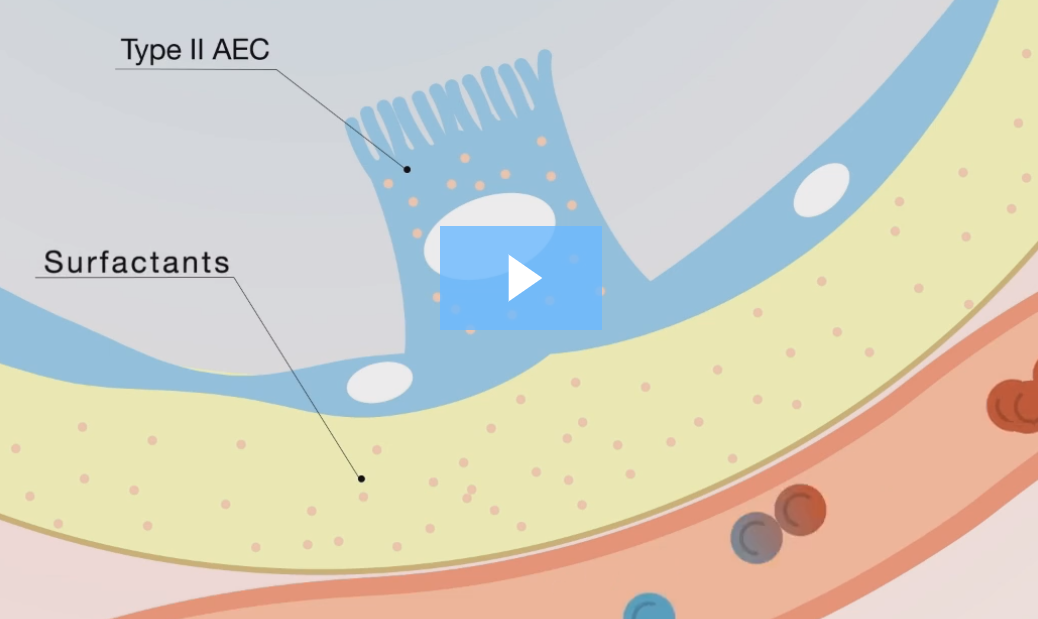
Krebs von den Lungen 6 (KL-6) is a mucin-like, high molecular weight glycoprotein expressed on the surface membrane of alveolar epithelial cells (AEC-II) and bronchiolar epithelial cells. It exists as a solubilized component in the pulmonary epithelial lining fluid by proteolytic cleavage.KL-6 serum concentration is elevated mainly because two mechanisms:
- Elevated pulmonary production due to diffuse hyperplasia of alveolar epithelial cells (AEC, called also pneumocytes)
- Increase in spillover to the systemic circulation due to leakage of the alveolo-capillary membrane
Watch our educational video
-
Clinical significance
More than 350 papers investigating the clinical significance of KL-6 in various types of Interstitial Lung Disease (ILDs) have been published suggesting that KL-6 serum levels are useful for:6- detecting the presence of AEC injury
- evaluating disease activity
- predicting clinical outcomes in various types of ILDs
KL-6 can be used as a prognostic marker since it has been shown that individuals with both, initial serum KL-6 >1000 U/mL and serial increases in serum KL-6 are associated with shorter survival, while those with both initial serum KL-6 <1000 U/mL and no serial increases in KL-6 were associated with longer survival.7
Besides, KL-6 may be helpful in other clinical conditions, such as the prediction of Acute Exacerbation (AE)8 or screening and monitoring of ILD associated with Connective Tissue Disease (CTD-ILD).9
-
References
- ATS / ERS. Am J Respir Crit Care Med 2002; 165: 277–304.
- Coultas DB, Zumwalt RE, Black WC, Sobonya RE. Am J Respir Crit Care Med 1994; 150:967-72.
- Luba Nalysnyk et al. Eur Respir Rev 2012; 21: 126, 355–361
- Koo SM and Uh ST. Korean J Intern Med 2017; 32:600-610
- Cottin V, Wollin L, Fischer A, et al. Eur Respir Rev 2019; 28: 180100
- Ishikawa N. et al. Respir Investig. 2012 Mar;50(1):3-13.
- Wakamatsu K et al. Respir Investig 2017, 55(1): 16-23
- Ohshimo S. et al. Respir Med. 2014 Jul; 108(7):1031-9
- Lee JS et al. Arthritis Res Ther. 2019 Feb 14; 21(1):58

Interstitial Lung Disease (ILD)
Millions of people suffer from lung diseases that affect any part of the respiratory system including the airways, the air sacs (alveoli), the interstitium, the blood vessels, and the pleura.
A large and heterogeneous group of diseases called Interstitial Lung Disease (ILD) affect the interstitium, a thin layer of cells between the alveoli, which contains blood vessels and cells that help support the alveoli, allowing efficient gas exchange.
